Air pressure is an essential component of our planet’s atmosphere, playing a crucial role in various weather phenomena.
It is defined as the force exerted by the weight of the air molecules above a certain point.
Understanding two primary concepts related to air pressure – relative air pressure and absolute air pressure – is vital for comprehending weather patterns and atmospheric dynamics.
Relative air pressure, also known as atmospheric pressure, is the measurement of air pressure compared to the surrounding environment. It is commonly expressed in units such as millibars or inches of mercury.
The term “relative” implies that the pressure is relative to the air pressure at sea level, which serves as a baseline measurement. The average atmospheric pressure at sea level is approximately 1013.25 millibars or 29.92 inches of mercury.
Absolute air pressure, on the other hand, refers to the actual pressure exerted by the air molecules without any comparison to the surrounding environment. Unlike relative air pressure, which considers the baseline pressure at sea level, absolute air pressure provides an absolute measurement at a specific location.
As the altitude increases, the air molecules become less dense, resulting in lower absolute air pressure – the precise equation is:

Relative pressure will therefore always be larger than absolute pressure unless you live at or below sea-level. Another simple way of looking at this is to imagine that each air molecule has a weight, then there will be more molecules stacked up on each other at sea level, than say at 1000 feet, and therefore more weight being borne (hence the higher number)
Here’s a few worked examples of the formual to show how the pressure falls with altitude:
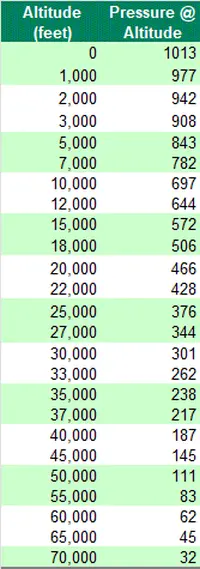
To compare pressure conditions from one location to another, meteorologists always use relative pressure so as to ensure consistency




[…] Here’s an easy to view monthly overview of the Barometric Pressure we’ve recorded in terms of average for the day as well as minimum and maximum, so you can easily see the variations across the years for the various months, as well as scanning the months of any particular year. The pressure we show is relative pressure (ie the equivalent pressure at sea level) rather than absolute pressure in order to allow proper comparability across locations – if you’d like to understand a bit more about this then please see this article […]